What is the difference between qualification and validation?
The knowledge area qualification answers basic questions on the distinction between qualification and validation, the qualification phases and the requirements for the storage and transport of medicinal products.
Here you will find answers to the following questions:
What is the difference between a qualification and a validation?
Qualification involves providing documented evidence that facilities, production and supply equipment, and premises are suitable for the intended purpose. Validation, on the other hand, provides evidence of the suitability of processes and procedures.
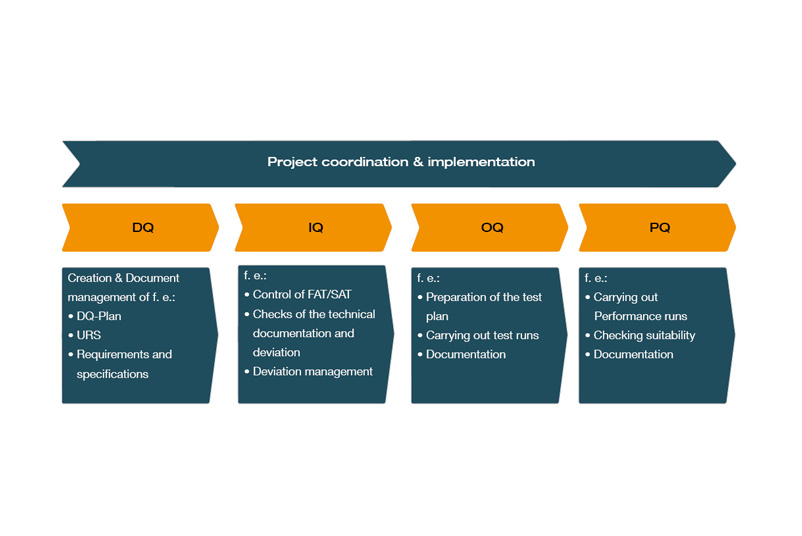
What are the qualification phases?
In the life cycle of a plant, different phases of qualification are distinguished:
- DQ: Design qualification
- IQ: Installation qualification
- OQ: Operational Qualification
- PQ: Performance Qualification
Do the high-quality requirements also apply to the storage and transport of medicinal products?
Yes, the high-quality requirements are firmly anchored in the Good Storage Practice (GSP) and the Good Distribution Practice (GDP). In its individual phases, the storage and transport process is influenced by quality risks of varying degrees, which must be adequately controlled. Qualification and validation measures can minimise these critical influencing factors and demonstrate compliance with GxP requirements.
What are the objectives of quality risk management according to ICH Q9?
The objectives are to identify, assess and reduce risks that could in any way negatively affect the quality of products and thus the safety of medicines. In this context, risk is defined as the combination of the probability of occurrence of a loss and its magnitude.