Validation concept for PRIMAS validated
For the validation of the test equipment management system PRIMAS validated we offer you a field-proven and standard-compliant validation package according to the specifications of GAMP® 5 and ensure the validated state over the entire life cycle.
In GxP-regulated areas (such as in the Pharmaceutical industry and the Medical technology) there are validation requirements for test equipment management systems over the entire life cycle. The validation of these systems is specified by various standards and regulations, e.g. DIN EN 13485, 21 CFR 820.72 or Annex 11 of the EU GMP Guide. The validation of computerized systems often ties up human resources and know-how in the GxP regulated company.
For the validation of PRIMAS validated, our industry experienced validation team provides you with the GxP compliant validation documentation and comprehensive know-how for your validation project at the same time.
Our service for you
- Validation of the test equipment management system PRIMAS validated
- Ensuring the validated state over the entire life cycle
- Validation report of the systemic validation at Testo Industrial Services
- Adaptation of the user-specific validation documentation incl. test documentation
- Comprehensive support during the entire validation project
- Fixed contact person during the entire validation project
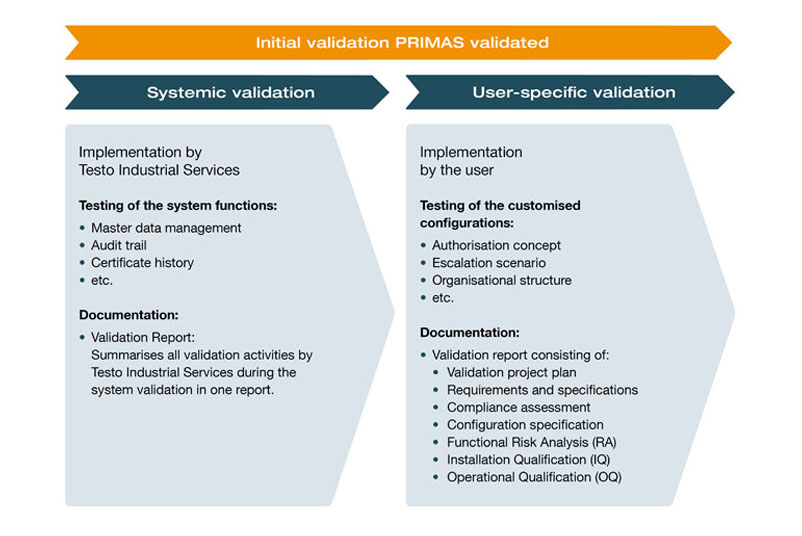
Initial validation
At PRIMAS validated is a validatable test equipment management system whose functionalities can be individually adapted to your requirements. Therefore, the validation includes both the systemic basis and its functions (such as audit trails and certificate histories), as well as your customer specific configurations (such as the escalation scenario and the authorisation concept). For the validation of PRIMAS validated we offer you a fully comprehensive validation service, consisting of a standardised validation concept supplemented by your user-specific configurations.
As part of our validation activities, we have subjected the systemic basis to comprehensive GAMP®-based risk management and implemented organizational and technical measures derived from this. The correct functionality was confirmed by extensive testing as part of the installation and functional qualification. A summary validation report documents all validation activities and provides you with additional security vis-à-vis the authorities in the event of an audit. The validation package is provided by the Validation Of your user-specific configurations. Our validation team creates the appropriate validation and test documents and adapts them to your configurations. The execution of the specified validation tests and their documentation is carried out by you or our validation support.
Change Management
In order to meet your requirements for an efficient and secure test equipment management solution, new functionalities are introduced into PRIMAS validated in an innovation cycle of 24 months at the earliest in the form of update packages. All changes and innovations go through a strict project planning and test phase and are already successfully used by users of the established PRIMAS online. We ensure the revalidation of the system via our change management.
We will inform you about the implementation date and the planned changes in good time before the update is published. In addition, depending on the scope of the changes, we provide you with validation documents with the necessary test documents, which you can integrate into your change management on site. With our uncomplicated revalidation services you benefit from a safe and controlled further development of your validated test equipment management system.

Your validation expert
Take advantage of my many years of experience in the implementation, conceptual design and coordination of validations/qualifications of computer-aided systems. For your questions around the validation of PRIMAS validated I am at your disposal - please contact me.

Further information

PRIMAS validated
The validated test equipment management solution for GxP-regulated areas

Expert interview
Our experts answer questions about PRIMAS validated

Test equipment management with PRIMAS
Holistic test equipment management solution for compliance with standards and directives